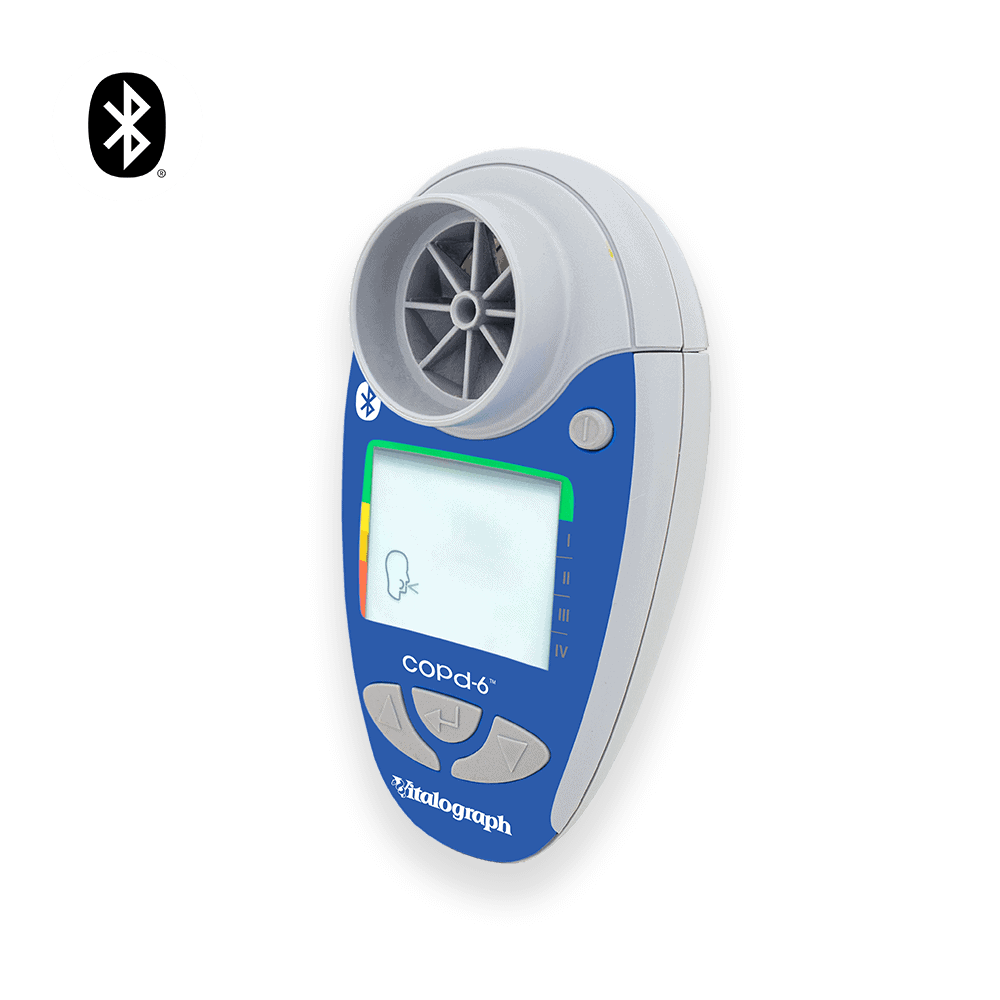
Vitalograph Copd-6 Copd Screening Device
For simple, fast & accurate COPD screening
The copd-6 identifies those at risk of COPD at the pre-symptomatic stage to allow early medical intervention and facilitate better clinical outcomes.
This pioneering device screens out those whose FEV1 is normal, and who therefore do not have COPD, without the risk of false COPD negatives, allowing spirometry resources to be focused on those most at risk.
€190.94
Shipping 1-2 Business Days
Trading for over 25 Years
The Vitalograph copd-6™ BT identifies those at risk of COPD at the pre-symptomatic stage, screening out those whose FEV1 is normal. The screener allows early medical intervention and facilitates better clinical outcomes.
Generates reports via Bluetooth connection and Vitalograph Reports software.
- Monitors COPD patients using their obstructive index – FEV1 as a percent of predicted
- Displays FEV1, FEV6, ratio, COPD classification and lung age
- Automatically sends data after each blow
- Can also send session data
- Large, easy-to-read display
One of our 4000 Series of respiratory monitors and screeners, the COPD-6 Monitor comes with:
- Pouch
- Instructions for Use
- Vitalograph Reports Software
- 2 x AAA Batteries
The Bluetooth® word, mark and logos are registered trademarks owned by Bluetooth SIG, Inc. and any use of such marks by Vitalograph is under license.
Features:
- For the early detection of COPD – quickly, simply and accurately;
- Identifies those at risk of COPD at the pre-symptomatic stage to allow early medical intervention and facilitate better clinical outcomes;
- Screens out those whose FEV1 is normal, and who therefore do not have COPD, without the risk of false COPD negatives;
- Facilitates ‘case selection’ so that spirometry resources can be focused on those most likely to be diagnosed with COPD;
- Monitors COPD patients using their ‘number’, the obstructive index, FEV1 as a percent of predicted;
- Displays FEV1, FEV6, ratio and % predicted, obstructive index, COPD classification and lung age;
- Built-in quality of blow indicator on a large, easy to read display;
- Easy to clean flowhead;
- Can be used with hygienic SafeTway mouthpieces or a BVF;
- Displays the GOLD COPD classification (stage I – IV) to help recognise the need for a change in the patient’s management plan;
- Requires only minimal instruction for use by non-respiratory specialists
| Product | Respiratory Monitor COPD-6 BT |
|---|---|
| Model | 4000 |
| Dimensions | 109mm (length) x 63mm (width) x 42mm (height) |
| Flow Detection Principal | Stator rotor |
| Accuracy | Better than ± 3% |
| Measurement Range | 0 – 9.99 L BTPS |
| Power Supply | 2 x 1.5V AAA batteries |
| Battery Life | 3 months of use, 3 tests per day |
| Quality Check | Slow start of test (Vext>5%) or a cough detected in the first second |
| FEV substituted for FEV6 | When FET < 3s and abrupt end of test |
| Performance standards: | ATS/ERS 2019, ISO 23747:2015, ISO 26782:2009 |
| QA/GMP standards | EN ISO 13485, FDA 21 CFR 820, MDSAP. |
| Communications | Bluetooth |
| PC Requirements for Vitalograph Reports | Windows® 7, Windows® 8 or Windows® 10 |
| Auto power down time | 2 minutes |
Related Documents:
Vitalograph-Telehealth-Brochure-4
- Hibernia Medical does not supply goods on a sale or return basis.
- Hibernia Medical does not accept the return of goods without prior written communication to the Customer Service department.
- Goods can be returned once approval in writing has been given by Hibernia Medical.
- A ‘Goods Return Form’ must be completed for all returns. Notification of the intent to return Goods must be made within 14 days of the Delivery/Invoice date.
- The only goods that will accepted for refund must be a current stock line and not a discontinued line, nonreturnable product or a special-order item.
- All approved goods for return must be in perfect condition, in original packaging, sellable and be completely without defect or damage. Such damage will totally cancel any obligation by Hibernia Medical for any refund whatsoever on the item.
- Electrical and photographic equipment will only be accepted if complete with all leads, accessories and software. Any software must have its original seal intact.
- Goods are returned at the sender’s responsibility and cost. Hibernia Medical accepts no responsibility for goods lost or damaged during return transit. We recommend that the returned item(s) to our office are registered/tracked. Additionally, Hibernia Medical does not refund return carriage, postage fees or insurance costs.
- Please check the package and label before opening any products to ensure that you have received the correct items. Shipment errors must be reported no later than seven (7) days after the invoice date.
- Non-stock items and special-order items are subject to a no-return policy.
- All sales of clearance items are final and we cannot accept returns.
Please Note: Special order items and discontinued items are not subject to this returns policy and will be dealt with on a case-by-case basis.
All items returned are subject to a restocking fee of 10%.
Items returned after 14 days are liable to additional handling charges:
- 14 – 21 days old 15% of invoice value or minimum of €20.00
- 21 – 30 days old 20% of invoice value or minimum of €20.00
- 31 – 60 days old 25% of invoice value or minimum of €20.00
- Returns after 60 days are dealt with on a case-by-case basis
If you return part or all of your order, we will not refund the delivery charge as this part of our service to you has been completed. In the cases of incorrect, damaged or faulty goods, the delivery charge for a replacement to be sent out will not be charged.
For returns/refunds requests within 14 days please contact our Customer Service Team on 01 8665727 or email [email protected]
If you’ve asked for a refund, we will credit the account you used to pay for your order. Please allow up to 14 days for the refund to appear on your account.
Our Address:
Hibernia Medical
E3 Calmount Business Park
Ballymount
Dublin 12
D12 NA40
You May Also Like

Bosch

Littmann

Saver One

Omron

Omron

Iem

Braun

and

riester

mesi

medi Plent

sono site

wench allyn

plinth medical